Osmosis Can Be Defined As
Osmosis Definition
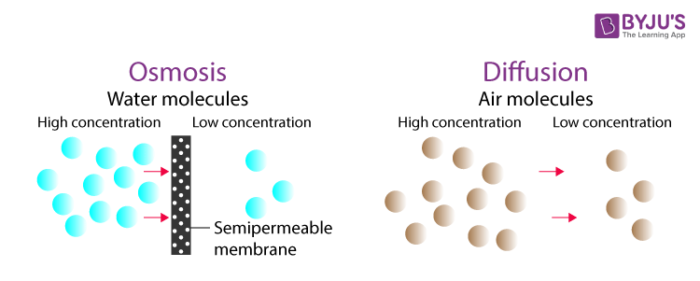
Osmosis is the diffusion of a solvent through a differentially permeable membrane . In biological systems, the solvent will usually be h2o. Osmosis will occur whenever the h2o concentrations are unlike on either side of a differentially permeable membrane.
Osmosis tin be defined equally the movement of water molecules from a higher h2o concentration expanse to the surface area of less water concentration through a semipermeable membrane. In other words, it can be defined as the diffusion of h2o molecules through a semipermeable membrane. Information technology is a special case of improvidence of water (High to depression). For example, water in the roots of plants is transported through osmosis.
What is Improvidence?
Improvidence is the internet move of molecules of a substance from a region of their higher concentration to a region of their lower concentration. Cyberspace movement ways in that location are more than molecules moving in ane direction than in the reverse direction.
Example: Opening a canteen of perfume in a room will effect in the gradual diffusion of the perfume from the region of higher concentration (the bottle) out into the room. Diffusion will go on until the perfume has a more than or less compatible concentration throughout the bottle and room.
The passive move of matter; the spontaneous tendency of a substance to move down its concentration, pressure level, or temperature gradient. Matter essentially moves (or diffuses) from an surface area of higher free energy to an area of lower energy until chemical equilibrium is achieved. In simple words, the movement of matter from high concentration area to low concentration area. For example, perfume diffuses into the air spreading the aroma.
Even though the process of diffusion and osmosis are almost similar, there are some notable differences betwixt them. Few key differences are listed below.
Difference between improvidence and osmosis:
| Osmosis | Improvidence |
| Information technology happens only in the liquid state. | It occurs in all states of matter i.eastward., solids, liquids or gases. |
| It should exist movement of only water or solvent through semipermeable membrane from lower concentration to higher concentration. | Any blazon of substance that moves from higher concentration area to lower concentration area. |
| It is applied only for the solvent part of the solution. | Improvidence is applied to all states of matter. |
| It requires semipermeable membrane. | This phenomenon does non require semipermeable membrane. |
| Osmosis depends on the rate of reduction of free free energy of one solvent. | It only depends on the free energy of the substance. |
| Influenced by the solute potential | Not influenced by solute potential |
| It is opposed by hydrostatic or turgor pressure. | Hydrostatic pressure or turgor pressure will non occur in diffusion. |
| In this process, concentration of solvent is non equalized. | Concentration of whole substance volition be equalized. |
| Factors like solute potential, h2o potential and force per unit area potential will not affect osmosis. | Factors similar solute potential, h2o potential and pressure level potential will not affect diffusion. |
Importance of Improvidence and Osmosis
Improvidence is essential for many organisms as it is a feature of a number of processes which control and supply vital substances to the body in society for basic survival. A few of these are discussed beneath. Gas exchange is one of these processes.
- Information technology is when much-needed oxygen is obtained by the torso in social club for respiration to have place and the waste product CO2 is taken out of the body. In united states of america mammals, the substitution takes identify in the lungs which comprise many alveoli.
- The importance of osmosis is to keep an equilibrium between the outside and inside environment.
- For instance, from the soil to the root hairs of the plant for the osmosis of water. The Soil has more concentration of water, therefore, it moves from soil to a region of depression concentration (ie. Plant)
Recommended Videos
Simple Improvidence
Read more:
- Characteristics of particles of matter
- Atoms and molecules
Osmosis Can Be Defined As,
Source: https://byjus.com/chemistry/diffusion-and-osmosis/
Posted by: coxninclow.blogspot.com




0 Response to "Osmosis Can Be Defined As"
Post a Comment